Share
Pin
Tweet
Send
Share
Send
In the journal "Chemistry and Life" No. 3 of 1972, there was an article on how to grow crystals of metallic copper, it is well written and I will not retell it, just give:
GROW COPPER CRYSTALS
No, we did not make a reservation. Not copper sulphate - you have probably received these crystals, and more than once, but real metal copper.
You also apparently observed very small crystals of copper - they cover an iron nail dipped in a solution of copper sulfate. They are so small that the reddish film seems solid, even. And in order to grow large crystals, you need to do this: slow down the reaction. When the molecules of the released substance will be deposited on ready-made small crystals, they will grow.
We will do so. At the bottom of a can or a chemical glass, put crystals of copper sulfate, cover them with fine table salt and cover with a circle of filter paper or blotter, cut exactly to the diameter of the vessel. On the paper circle we put a circle a little smaller, this time iron. It must be cleaned with a fine emery cloth. And finally, fill everything with a saturated solution of sodium chloride so that it is several centimeters above the iron circle. You, of course, understood why table salt was needed. That's right - to slow down the release of copper.
The preparation is completed, the experience itself begins. He will go without our intervention, we only need to wait and observe. How long to wait depends on the conditions of the experiment, primarily on temperature. Usually after a few days, shiny copper crystals form. Their shape and size can be different depending on the size of the crystals of copper sulfate and their amount, on the diameter of the vessel, the height of the salt layer, temperature. Sometimes beautiful copper "trees" grow - dendrites (the word comes from the Greek "tree"), crystals unfinished in development. Snowflakes, hoarfrost, frosty patterns on glass are also dendrites.
Of course, you will want to save the resulting crystals. Rinse them with water, fill with diluted sulfuric acid and keep in a sealed container, without air.
I. Ilyin.
The thicker the layer of salt, and the lower the temperature, the slower and, therefore, larger and more shaped crystals will grow. Also, the size of the crystals and the growth rate will depend on the size of the crystals of salt, because the crystals grow in a layer of salt, between its crystals. Salt and copper sulphate, cheap and readily available compounds, and it seems to me very interesting to conduct a study to identify optimal growth conditions and obtain regular and large crystals.

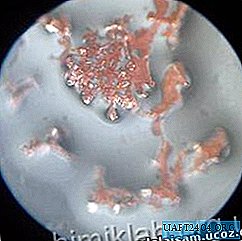
Source: himiklab.org.ua
Share
Pin
Tweet
Send
Share
Send











